A Little Background
 While many in industry regard Safety Data Sheets (SDS) as a tedious formality—a document that is seldom read—the information in an SDS can have very serious downstream effects, especially if it’s inaccurate.
While many in industry regard Safety Data Sheets (SDS) as a tedious formality—a document that is seldom read—the information in an SDS can have very serious downstream effects, especially if it’s inaccurate.
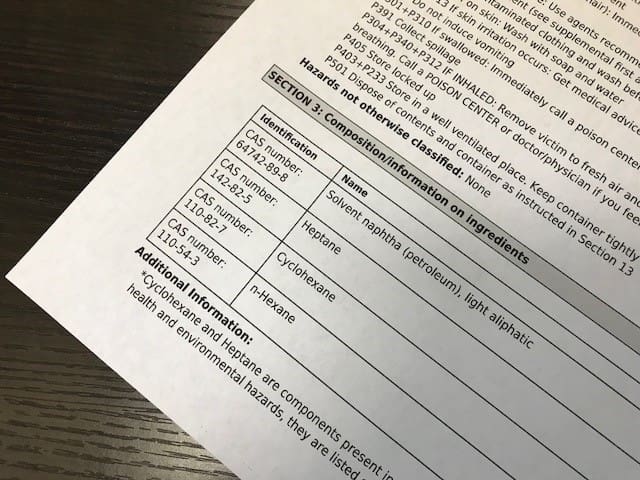
Something as seemingly benign as a supplier listing the wrong Chemical Abstract Services (CAS) number in Section 3 of the SDS, can lead downstream users (DU) importing that substance to say, the European Union, to register the wrong substance, purchase the wrong Letter of Access, disrupting their supply chain, and delaying their ability to place product on the market. Once this error is traced back to the SDS, you can bet that the DU organization will seek damages for the inaccurate CAS number listed on the supplier’s SDS.
But How is This Possible?
Let’s take zinc distearate, CAS 557-05-1, for example. Zinc distearate is a common mold release agent, used to prevent plastics from sticking to the molds that they’re created within. Zinc distearate is derived from either animal fats or vegetable oils and is often placed on the market as a mixture containing a range of fatty acid salts, from zinc palmitate, to zinc myristate, etc., but with the majority constituent being zinc distearate. Many often refer to these mixtures simply as “zinc distearate,” giving the false impression that they contain 100% zinc distearate.
The Impact
Downstream users of zinc distearate, not being experts in its synthesis or composition, may sometimes forget that this “substance” can contain significant amounts of other fatty acid salts, as listed above, and is really a mixture of substances, in some cases, better described by CAS 91051-01-3 “fatty acids, C16-C18, zinc salts,” than by CAS 557-05-1 “zinc distearate.” In fact, designation of zinc distearate as “CAS 557-05-1” requires a minimum content of 80% zinc distearate.
These details matter an awful lot to regulatory staff who are responsible for developing the meticulous regulatory dossiers of a substance, which can take months of effort, dozens of tests, lengthy summaries and interpretations, etc., to complete—only to find that these results were generated for the wrong substance, and now need to be repeated, to the tune of hundreds of thousands of dollars! And it gets worse: if the DU organization has already exceeded 1 metric ton of the substance placed on the market in the EU, for example, then all import activity of that product or substance must stop until registration is complete—the lost revenue due to this regulatory delay can be devastating to an organization! Those authoring SDS had better get these seemingly small details right, the first time.
With a broad team of experts having years of experience authoring SDSs globally, Global Safety Management is programmed to pay attention to the details that matter most. Need help with compliance? Get in touch today!